Why Specific heat at constant pressure is greater than Specific heat at constant volume?
First of all, let us define Specific Heat at Constant Pressure (Cp) and Specific Heat at Constant Volume (Cv):
Specific Heat at Constant Pressure (Cp):
Specific heat at constant pressure is deImportant interview questions about mechanical engineering – Thermodynamics (Part II)fined as the amount of heat required to raise/increase the temperature of a gas/substance having unit mass by one (degree C) keeping the pressure constant. Unit: J/kgK
Specific Heat at Constant Volume (Cv):
Specific heat at constant volume is defined as the amount of heat required to raise/increase the temperature of a gas/substance having unit mass by one (degree C) keeping the volume constant. Unit: J/kgK
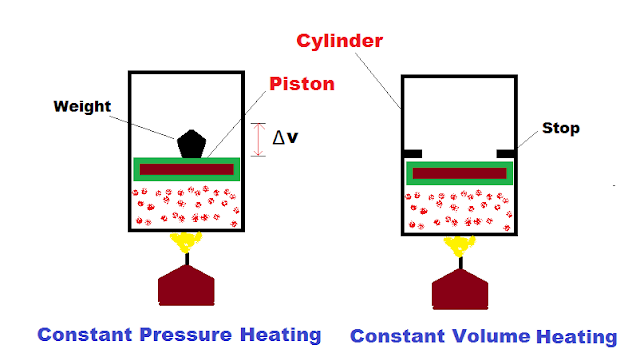
Now consider the following experimental setup having piston-cylinder arrangement containing gas heated by an external heat source.
When we keep some fixed Weight on the piston, the piston will exert some pressure on the gas. When gas is heated, it will expand and does external work on the piston i.e. (PdV) (see figure). So, some portion of heat is wasted in doing external work, and some part in increasing internal energy (which is proportional to temperature). Thus, more amount of heat (Cp) is required to raise the temperature through unit amount.
While in the second case, when we do not allow the piston to move upward, no external work is done on position and all the external heat is utilized to raise the temperature of gas rather than sharing the heat with external work. Thus, Cv is less in amount compared to Cp.
Thus, the difference between Cp and Cv is always greater than zero and is called ‘Gas constant’.
Significance of the compressibility factor
The compressibility factor ( Z = PV/mRT) distinguishes real gas from its ideal gas behavior. When the value of Z is more than one, it signifies the real gas behavior.